Using a Diagram Describe the Bonding in Zinc
1 ii Draw a dot-and-cross diagram to show the bonding in barium oxide. Show outer electrons only.
Chem4kids Com Zinc Orbital And Bonding Info
Here a is brittle b is partially ductile and c is completely ductile in nature.

. Up to 24 cash back Zinc oxide is reduced by carbon which takes away _____ to leave zinc metal. Heres an example using sodium and chlorine. The zinc sulide in the ore is irst converted into zinc oxide.
For example covalently bonded gallium atoms tend to form crystal structures that are held together via metallic bonds. Describe the structure and bonding in a metallic element. This seems to indicate that cohesion in these metals differs considerably from the normal nondirectional metallic bonding.
When zinc chloride is molten it will conduct _____. Its ion is of similar size and its only common oxidation state is 2. ZnO2 its an ionic bond because it is between a metal and a non metal using orbital notation diagram the formation of an ionic bond between aluminum and fluorine ions attract to form AlF3 using electron configuration diagram the formation of an ionic bond between barium and nitrogen Ba3N2.
1 ii Explain why zinc ions move towards the negative electrode. It is the first element of group 12 of the periodic table. They are electrical conductors because their delocalised electrons carry electrical charge through the metal.
The atomic weight of silver is 10786 gmol. Do not use staples paper clips glue or correction luid. You will often see electrons drawn like.
2 iii Calculate the number of barium ions in 150 g. You may use an HB pencil for any diagrams or graphs. 1 Barium combines with oxygen chlorine and nitrogen to form ionic compounds.
I State what is meant by the term ionic bond. Draw a dot and cross diagram using outer electrons only to show how zinc chloride ZnCl 2 is formed from zinc and chlorine atoms. Magnitude of positive charge held by the metal cation.
DO NOT WRITE IN ANY BARCODES. In some respects zinc is chemically similar to magnesium. People who have a zinc deficiency can take hydrated zinc sulfate ZnSO 4xH 2O as a dietary.
Either one works but the dust produces better results. Steel that has been dipped into molten zinc to prevent rusting and so have visible zinc crystals on its surface. Attention is directed to the very large departures of the structures of the zinc family IIb metals from those produced by the close packing of spheres.
2 b Zinc is an essential trace element. Use the diagram to explain why iron from the blast furnace is hard and brittle. In this example the electrons are shown as dots and crosses.
Describe the structure and bonding in a metallic element. You may use a diagram to illustrate your answer. Metallic dusts are a flammable hazard so caution is warranted.
Do not use staples paper clips glue or correction fluid. The Reaction between Magnesium and Oxygen. The atomic number of element Pis11 and that of Q a Write down the possible formula of the compound formed between Pand b Using dots and crosses x to represent electrons draw a diagram to represent the bonding in the compound ina above 24.
3 iv Suggest why the introduction of a different atom into the structure makes the alloy less malleable than the pure metal. 2 With the aid of a diagram describe and explain in terms of the electronic structure of each element the bonding in magnesium chloride. Include a labelled diagram and any appropriate charges in your answer.
Question 1 15 marks Calculate the number of atoms in 1000 kg of silver. Hot air is blown into the bottom of the furnace. Describe how zinc oxide is made from zinc sulfide.
An oxygen atom will gain 2 electrons to form a stable 2-ion. Magnesium is in group 2 of the periodic table. I Name the product formed at the positive electrode.
The zinc sulfide in the ore is first converted into zinc oxide. A magnesium atom will lose 2 electrons to form a stable 2 ion. Describe the structure and bonding in a metal.
13 a Zinc reacts with chlorine to form the ionic compound zinc chloride. State three chemical properties of transition elements. Write your answers in the spaces provided in the Question Paper.
The mercurous ion also exhibits metallic and covalent bonding. Describe how zinc oxide is made from zinc sulide. A Zinc chloride is an ionic substance.
It is proposed that there is a system of covalent bonds in the basal plane. Describe how zinc oxide is made from zinc sulfide. Section A Answer all questions.
State one other large scale use of zinc. 1 11 UCLES 2014062031MJ14Turn over. 2 b Zinc oxide is converted into zinc.
1 ii Write a chemical equation for the reaction in ai. Zinc oxide and coke are fed into a furnace. 3 b Put the three metals copper nickel and zinc in order of reactivity.
Most reactive Least réactive 1 c Zinc is a metal in transition elements group Describe the bonding in zinc. Metals are good conductors of electricity because the electrons in the electron sea are free to flow and carry electric current. Name the type of bonding and structure found in.
Ductility is property of metals for what one can apply stress onto a metal to make it longer or wider without breaking. 2 OCR 2017 Answer all the questions. Dot and cross diagrams help us to model when ions are formed from atoms.
Do not use staples paper clips glue or correction fluid. 1 iii Describe the bonding in a typical metal such as zinc and then explain why it is malleable. Metals are ductile and malleable because local bonds can be easily broken and reformed.
The factors that affect the strength of a metallic bond include. Zinc has a melting point of 420 C and a boiling point of 907 C. B Zinc ions move towards the negative electrode where they gain electrons to produce zinc.
You may use an HB pencil for any diagrams or graphs. - a Ice b Magnesium chloride 2 is8 Q 25. Section B Answer any three questions.
On the following equation draw a ringaround the reducing agent and an arrowto show the change which is oxidation. Zinc is the 24th most abundant element in the. Zn 2Ag.
1 ii The melting points and boiling points of lead and zinc are given in the table. Total number of delocalized electrons. Write your answers in the spaces provided in the Question Paper.
A Barium oxide BaO has a giant ionic lattice structure. Use about 25 mL of solution per 250 mL beaker and cover the bottom with zinc. 3 i With the aid of a diagram s describe all the primary and secondary bonds.
The structure and bonding of metals explains their properties. Zinc Zinc in commerce also spelter is a chemical element with symbol Zn and atomic number 30. They form ions which bond.
Place the cleaned pennies on top of the granular zinc in the hot zinc chloride solution. 2 b Zinc metal is made by the reduction of zinc oxide. Oxygen is in group 6 of the periodic table.
You may use an HB pencil for any diagrams or graphs. Zn Pb22 Zn Pb 2 iiiComplete the following ionic equation. Granular zinc can be used instead of zinc dust.
The metallic bonding electron sea model can explain the physical properties of metals. The characteristics of metallic bonds explain a number of the unique properties of metals. Your answer should describe the structure and bonding of a metal 4 marks Metals have a giant structurelattice 1 mark made up of positive ions surrounded by delocalised electrons 1 mark with.

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons
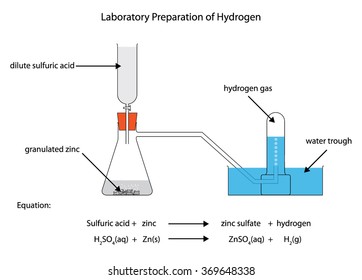
Labeled Diagram Laboratory Preparation Hydrogen Zinc Stock Vector Royalty Free 369648338

The Molecular Orbital Diagram Of Mixing Of A Zn Atom And As 73 A Download Scientific Diagram
No comments for "Using a Diagram Describe the Bonding in Zinc"
Post a Comment